Raw Material Collection
Test Parameters
- Microbiological Contamination (Test Per Batch): Salmonella; Listeria
- Chemical Residues (Test Per Batch): Antibiotic; Heavy Metal
Standards & Methods
- Listeria: FDA BAM Ch.10 (Chromogenic agar); <10 CFU/g
- Salmonella: ISO 6579-1:2017 (Rapid PCR); Negative in 25g
- Antibiotics: HPLC (EU Regulation 37/2010); Tetracyclines: ≤0.01 ppm
- Heavy Metals: ICP-MS (EPA 6020B); Lead: ≤0.1 ppm
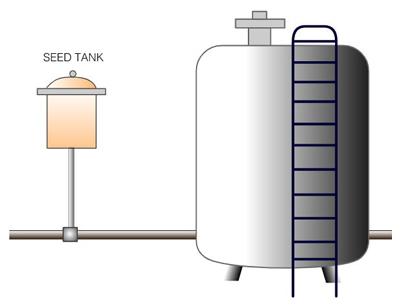
Fermentation
Test Parameters
- pH & Acidity (Test Per Hour)
- Stain Viability (Test Per 2 Hour)

Standards & Methods
- pH: Potentiometry (ISO 7238); pH: 4.0-4.3
- Lactic Acid: Titration (AOAC 967.21); 1.5-2.0% w/w
- Stain Viability: Flow Cytometry (ISO 19344:2015)
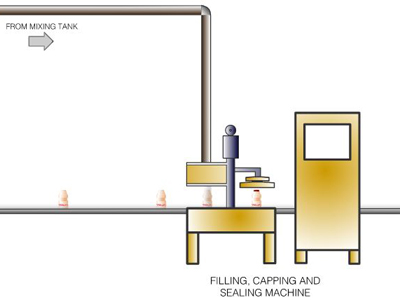
Filling & Sealing
Test Parameters
- Seal Integrity (Test Per 30 Minutes)
- Fill Volume (Continuous monitoring)
Standards & Methods
- Seal Integrity: Vacuum Decay Test (ASTM F2338), No leakage at 0.3 bar vacuum; Dye Penetration (FDA 21 CFR 177.1210), Zero dye ingress
- Fill Volume: Gravimetric Check (ISO 9001:2015),65mL ±0.5mL (Original); Automated Vision System (Mettler Toledo), 80mL ±0.5mL (Light)
Cold Chain
Test Parameters
- Cold Chain Temperature (Test Per Batch)
- Post-Distribution Strain Count (Test Per Month)

Standards & Methods
- Cold Chain Temperature: RFID Temperature Tags, 2-6°C (no excursions >15min)
- Post-Distribution Strain Count: Random Retail Sampling (ISO 11133), ≥80% labeled CFU at point of sale
